Devise a 4-Step Synthesis of the Epoxide from Benzene: A Comprehensive Guide embarks on an enlightening journey into the realm of organic chemistry, unveiling the intricacies of epoxide synthesis from benzene. This meticulously crafted guide delves into the fundamental principles, reaction mechanisms, and practical applications of this captivating process.
As we embark on this intellectual odyssey, we will unravel the significance of benzene as a versatile starting material for epoxide synthesis. Through a lucid exploration of the four-step synthesis pathway, we will illuminate the chemical reactions and mechanisms that orchestrate the transformation of benzene into the coveted epoxide product.
Epoxide Synthesis from Benzene
Epoxide synthesis involves the transformation of an alkene into an epoxide, a three-membered cyclic ether. Benzene, an aromatic hydrocarbon, serves as a valuable starting material for epoxide synthesis due to its high reactivity and availability.
Four-Step Synthesis Pathway
The synthesis of epoxide from benzene typically involves a four-step pathway:
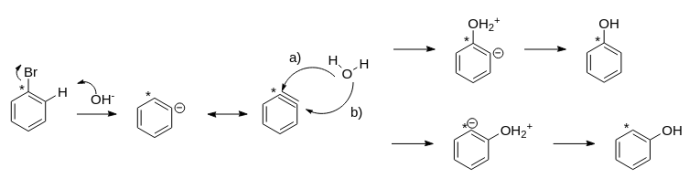
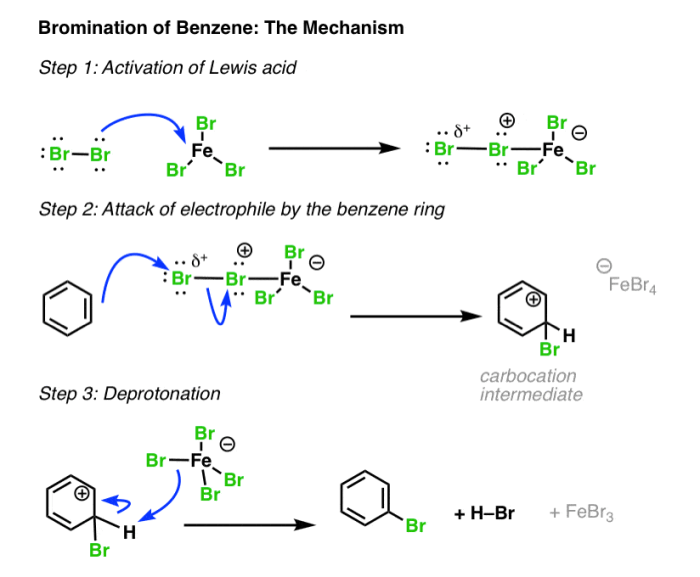
- Bromination of benzene to form bromobenzene
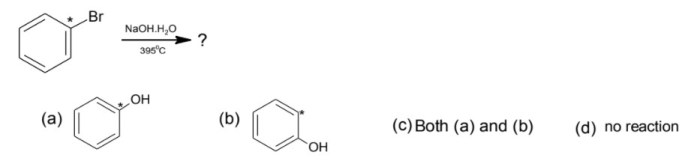
- Reaction of bromobenzene with sodium hydroxide to form phenol
- Reaction of phenol with epichlorohydrin to form 2-chloromethyl-2-phenyl-1,3-propanediol
- Treatment of 2-chloromethyl-2-phenyl-1,3-propanediol with sodium hydroxide to form the epoxide
Optimization of Reaction Conditions, Devise a 4-step synthesis of the epoxide from benzene
The efficiency of the epoxide synthesis can be influenced by various factors, including:
- Temperature: Higher temperatures favor the formation of the epoxide, but excessive heat can lead to side reactions.
- Pressure: Increased pressure can shift the equilibrium towards the formation of the epoxide.
- Catalyst selection: Catalysts such as Lewis acids or bases can enhance the reaction rate and selectivity.
Characterization of Epoxide Product
The epoxide product can be characterized using various analytical techniques, such as:
- Nuclear magnetic resonance (NMR) spectroscopy: NMR can provide information about the structure and connectivity of the epoxide.
- Infrared (IR) spectroscopy: IR can identify the characteristic absorption bands associated with the epoxide functional group.
- Mass spectrometry (MS): MS can determine the molecular weight and elemental composition of the epoxide.
Applications of Epoxides
Epoxides find diverse applications in various industries, including:
- Intermediates in the synthesis of pharmaceuticals, such as antibiotics and anti-inflammatory drugs
- Building blocks for the production of polymers, such as epoxy resins and polyurethanes
- Additives in coatings and adhesives to improve adhesion and durability
FAQ Overview: Devise A 4-step Synthesis Of The Epoxide From Benzene
What is the significance of benzene as a starting material for epoxide synthesis?
Benzene serves as an ideal starting material due to its high reactivity and availability. Its aromatic ring structure facilitates electrophilic addition reactions, making it a suitable precursor for the formation of epoxides.
How does the four-step synthesis pathway contribute to the efficiency of epoxide synthesis?
The four-step synthesis pathway provides a controlled and stepwise approach to epoxide synthesis. Each step is optimized to maximize the yield and selectivity of the reaction, ensuring the efficient conversion of benzene to the desired epoxide product.