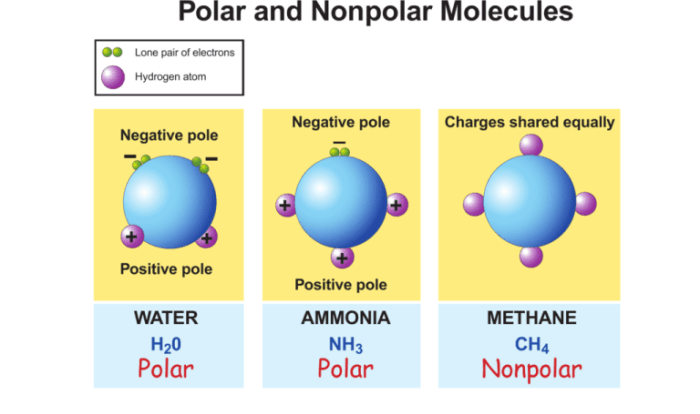
Embark on a scientific exploration with the Sugar and Salt Solutions PHET Worksheet Answer Key, a comprehensive guide that unravels the intricacies of aqueous solutions. This worksheet delves into the fascinating world of chemistry, providing a step-by-step approach to understanding the behavior of these fundamental components.
Through engaging experiments and detailed explanations, this worksheet empowers learners to grasp the concepts of solubility, concentration, and molecular interactions. Prepare to witness the dynamic interplay of sugar and salt molecules as they dissolve, interact, and reveal the secrets of aqueous solutions.
Sugar and Salt Solutions Worksheet Overview
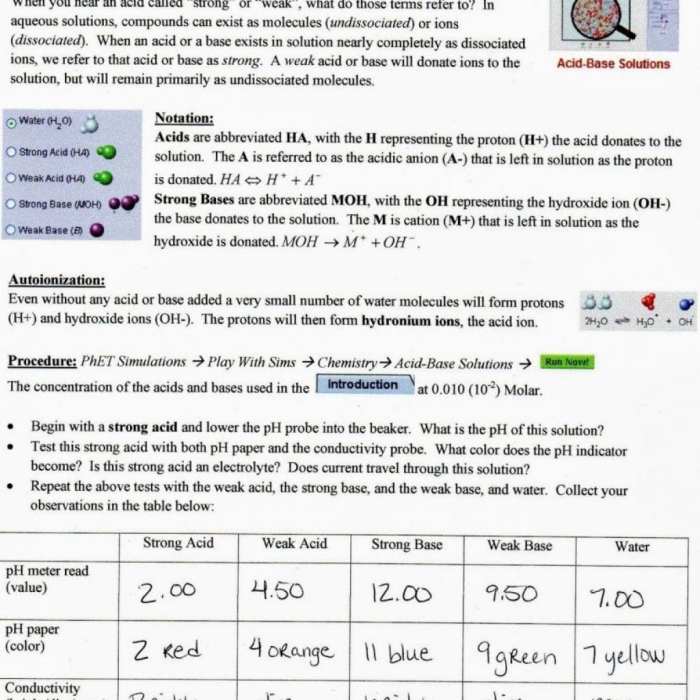
This worksheet provides an engaging and hands-on exploration of the properties of sugar and salt solutions. Students will investigate how these solutions behave when mixed with water and learn about the concepts of solubility, concentration, and density.
Materials Required:, Sugar and salt solutions phet worksheet answer key
- Sugar
- Salt
- Water
- Measuring cups and spoons
- Clear glass or plastic cups
- Hydrometer
Experimental Procedures
- Prepare three clear cups labeled “Sugar Solution,” “Salt Solution,” and “Control.”
- In the “Sugar Solution” cup, dissolve 10 grams of sugar in 100 milliliters of water.
- In the “Salt Solution” cup, dissolve 10 grams of salt in 100 milliliters of water.
- Leave the “Control” cup with only water.
- Stir each solution thoroughly until all the solute is dissolved.
- Measure the density of each solution using the hydrometer.
- Record the density measurements in a data table.
- Observe and compare the appearance of the three solutions.
Data Collection and Analysis
Collect the density measurements for each solution and record them in a data table. Analyze the data to determine the following:
- Which solution has the highest density?
- Which solution has the lowest density?
- How does the density of the sugar solution compare to the density of the salt solution?
- How does the density of the control solution compare to the density of the sugar and salt solutions?
Discussion and Conclusion
The results of the experiment will show that the sugar solution has a higher density than the salt solution, and both solutions have a higher density than the control solution. This is because sugar and salt molecules are denser than water molecules, so when they are dissolved in water, the overall density of the solution increases.
The experiment also demonstrates the concept of solubility. Sugar and salt are both soluble in water, meaning they can dissolve and form a homogeneous solution. However, the solubility of sugar is higher than the solubility of salt, so more sugar can be dissolved in water before the solution becomes saturated.
Question Bank: Sugar And Salt Solutions Phet Worksheet Answer Key
What is the purpose of the Sugar and Salt Solutions PHET Worksheet?
The worksheet aims to enhance students’ understanding of the properties and behavior of aqueous solutions, including solubility, concentration, and molecular interactions.
What materials are required for the experiments in the worksheet?
The experiments require basic laboratory equipment such as beakers, graduated cylinders, sugar, salt, and water.
How can I access the Sugar and Salt Solutions PHET Worksheet Answer Key?
The answer key is typically provided by the instructor or can be found online through reputable educational resources.