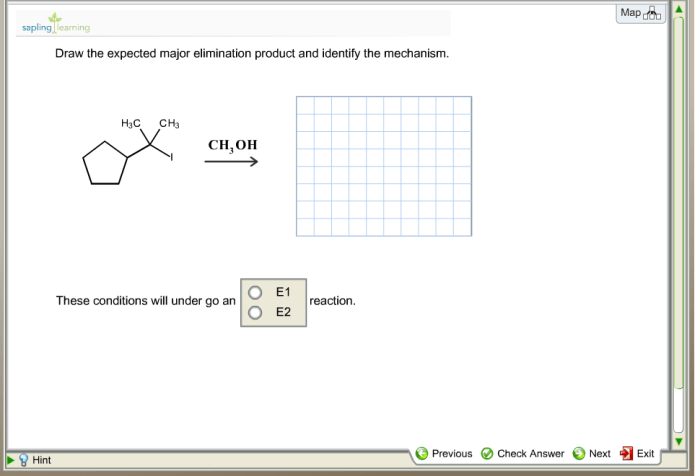
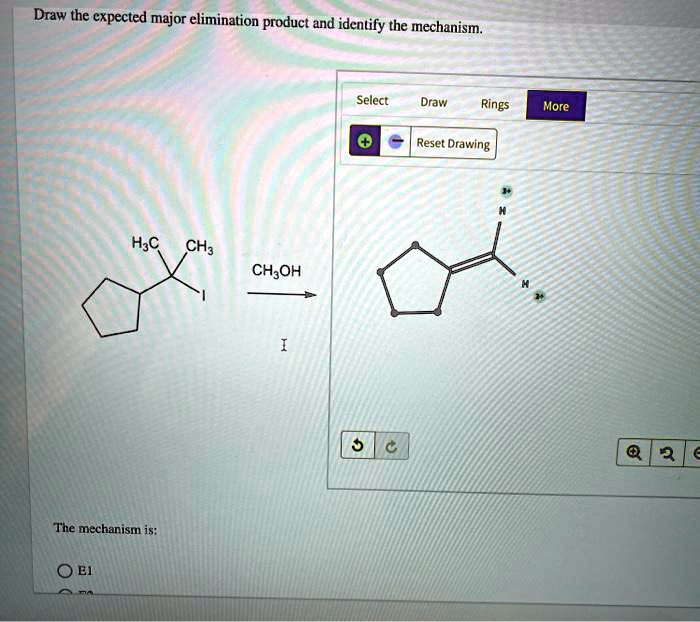
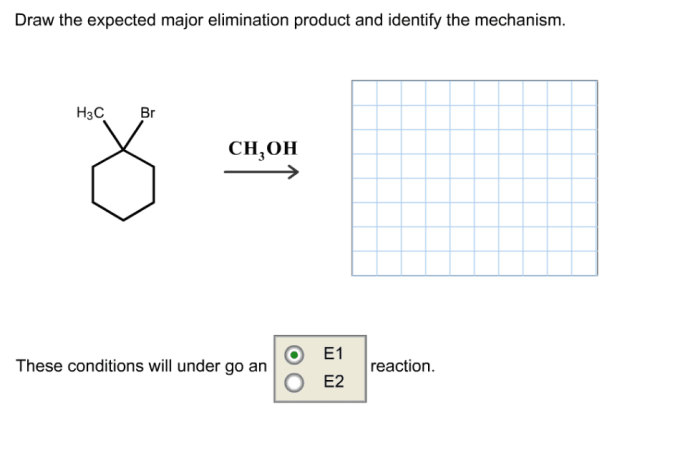
Draw the expected major elimination product and identify the mechanism. Elimination reactions are a fundamental class of organic reactions that involve the removal of two groups from a molecule, typically a proton and a leaving group. These reactions are widely used in organic synthesis for the construction of alkenes and alkynes.
Understanding the mechanism of elimination reactions is crucial for predicting the regioselectivity and stereoselectivity of the reaction, which are important considerations in organic chemistry.
This guide will provide a comprehensive overview of elimination reactions, including the different types of elimination reactions, the factors that influence the regioselectivity and stereoselectivity of the reaction, and the role of the base and the leaving group in the reaction mechanism.
We will also provide examples of elimination reactions with varying substrates to illustrate the different types of elimination products that can be obtained.
Elimination Reactions: Draw The Expected Major Elimination Product And Identify The Mechanism
Elimination reactions are organic reactions that involve the removal of two groups from a molecule, typically a proton (H+) and a leaving group (LG), resulting in the formation of a double bond. Elimination reactions are classified into two main types: E1 (unimolecular elimination) and E2 (bimolecular elimination), based on their mechanisms.
Draw the Expected Major Elimination Product
To draw the expected major elimination product, follow these steps:
- Identify the most substituted carbon atom adjacent to the leaving group.
- Remove the proton from the carbon atom that is antiperiplanar to the leaving group.
- Remove the leaving group.
- Form the double bond between the carbon atoms from which the proton and leaving group were removed.
Identify the Mechanism
The mechanism of an elimination reaction depends on the type of reaction (E1 or E2) and the substrate.
E1 Mechanism
- Proton removal occurs first, forming a carbocation intermediate.
- The leaving group then departs from the carbocation, forming the double bond.
E2 Mechanism
- Proton removal and leaving group departure occur simultaneously in a concerted step.
- The base abstracts the proton, while the leaving group departs.
Provide Examples, Draw the expected major elimination product and identify the mechanism
Examples of elimination reactions include:
- Dehydration of alcohols to form alkenes
- Dehydrohalogenation of alkyl halides to form alkenes
- Elimination of ammonia from ammonium salts to form alkenes
Compare E1 and E2 Reactions
The following table compares the characteristics of E1 and E2 reactions:
| Characteristic | E1 | E2 |
|---|---|---|
| Mechanism | Unimolecular | Bimolecular |
| Rate law | First order | Second order |
| Product distribution | Zaitsev’s rule | Hofmann’s rule |
| Stereochemistry | Not stereospecific | Stereospecific (anti-periplanar) |
Query Resolution
What is an elimination reaction?
An elimination reaction is a chemical reaction in which two groups are removed from a molecule, typically a proton and a leaving group.
What are the different types of elimination reactions?
There are two main types of elimination reactions: E1 reactions and E2 reactions. E1 reactions proceed through a carbocation intermediate, while E2 reactions proceed through a concerted mechanism.
What are the factors that influence the regioselectivity and stereoselectivity of an elimination reaction?
The regioselectivity and stereoselectivity of an elimination reaction are influenced by a number of factors, including the structure of the substrate, the strength of the base, and the reaction conditions.